Benchmark Guidance Values for Microbiological Monitoring on Surfaces: a Literature Overview
Raffaella Giovinazzo,1* L. Caradonna,1 G. Giaquinta,1 E. Guerrera,1 M. Mameli,1 A. Mansi,2 G. Marena,1 T. Mastromartino,1 D. Sarto,1 P. Tomao2
1 Inail – Technical Advisory Department for Risk Assessment and Prevention, Rome, Italy
2 Inail – Department of Occupational and Environmental Medicine, Epidemiology and Hygiene, Rome, Italy.
Introduction
The Directive 2000/54/EC1 of the European Parliament and of the Council of 18 September 2000 on the protection of workers from biological risks is geared toward workers exposed to microorganisms, cell cultures and human endoparasites during their professional activities. Environmental microbiological monitoring is not explicitly mentioned, but it is implicitly asserted in the Article 64 and in the Annex IV, point 1.3 of the Italian Legislative Decree 81/2008,2 with the oblige of the employer to clean regularly the work areas, installations and mechanisms and to ensure “adequate” hygiene conditions. The lack of widely accepted quantitative thresholds for acceptable levels of microbiological contaminants prevents adequate assessment of the hygienic quality of indoor environments.
The aim of this paper is to present a literature overview of the main microbiological environmental monitoring techniques and related benchmarks proposed for the assessment of the hygienic status of surfaces in different workplaces. The bibliographical research has been carried out mainly using the following key words: surface, (micro) biological contamination, surface sampling, surface contamination, hospital surface contamination, indoor surface microbiological pollution. Articles in Italian, English and French, from 1981 until now, have been examined, including those regarding the surfaces of Personal Protective Equipment (PPE).
Literature Review
Procedures for the assessment of the hygienic status of surfaces
For microbiological monitoring of surfaces there are classic methods, such as contact plates, sponge-bag, swabs, as well as technical biochemistry tests like ATP bioluminescence. Among the microbiological techniques, the sterile contact plates (RODAC – Replicate Organism Direct Agar Contact) are often used as a single technique or in combination with others. This method seems to be more sensitive to detect Gram-positive bacteria compared to Gram-negative ones on hospital environment surfaces but it cannot be used for uneven surfaces or awkward areas e.g. door handles, curved surfaces and rough surfaces. The contact plate technique is used to monitor the surfaces of “clean room” of the pharmaceutical sector,39 of sanitary rooms and domestic environments,23 and for the evaluation of the microbiological contamination of antique books and manuscripts.27 This technique has been used, also for the evaluation of the sterility of personal protection system.40
The swabs allow the microbiological sampling of hard surfaces, for example, behind the sinks41 and the pipes42 or along the bedrails.43 This method is chosen to sample smooth, non-porous surfaces, like steel, painted walls, floor tiles, laminated wood etc. Sterile swabs are made of several materials: cotton, rayon,44 nylon45 and polyurethane foam.46,47,48 The nylon swabs allow a greater efficiency of recovery of the microbial cells, because the microorganisms do not penetrate the nylon matrix, as occurs in the cotton swabs, and they remain on the external surface. The nylon swabs, compared to rayon, have a greater sensibility and a greater ability to recover S. aureus cells from the clinical patient’s skin49,50 and greater sensibility also when used in environmental sampling.51 The use of swabs is widely diffused in the food sector, in Good Manufacturing Practices (GMPs) and Hazard Analysis Critical Control Point (HACCP) programs, in healthcare sector52 for detection of fungi and Gram-negative bacteria53 and in hospital kitchens during food manipulation.54 The sponge-bag method uses a sponge composed of an absorbent sterile material contained in a sterile bag that can easily be closed. The method is widely used for the evaluation of the hygienic status of the surfaces in the food sector. Compared to the swab method, this method has the advantage of allowing the collection on wider surfaces and to ensure a greater collection efficiency in the presence of biofilm or cracks, because it is possible to make more pressure. This method is not suitable for small surfaces. The use of the sponge has been also validated for the detection, recovery and quantification of vital spores of Bacillus anthracis inoculated on steel surfaces, in environmental contamination simulations.55 RODAC plates, swabs and sponge-bag are used to obtain both qualitative and quantitative analyses. The bioluminescence ATP technique is used as initial screening method to monitor the level of cleanliness of the surfaces in several workplaces, in particular in the field of HACCP. This technique does not allow the differentiation between species of bacteria and/or molds, but it gives a rapid detection of the contamination level.56,57 It must be integrated with classical microbiological tests.58-59 In hospitals, this technique may provide additional information of cleaning efficacy and allow identification of environmental surfaces that require additional cleaning. This technique is often applied in the pharmaceutical industry60 and in cosmetic industry.61
Legislation
Technical regulations or guidelines on microbiological control of surfaces are not available. However, several regulations and documents of national and international scientific organizations carried out microbiological tests on surfaces in the pharmaceutical,28-30 health-care31-33 and food/feed for animals34,35,36 sectors. Some documents set the requirements for the environments of sterile products manufacture, and define specific classes of microbiological contamination, according to the level of environmental cleanliness required.28,30,37 General indications or operative criteria for the environmental microbiological survey can be found in all these documents,33,38 as well as contamination intervals useful for the classification of specific work environments and microbial contamination indicators to be used as references, for the assessment of the hygienic level of the surfaces in relation to the work context.31,32
Microbial contamination in workplace
In the assessment of biological agents in workplaces, the monitoring of airborne and surface microbiological contamination is an important step. Airborne biological agents in the workplaces can be deposited on the surfaces that act as substrate for the proliferation and the diffusion of microorganisms in the environment.
Recently, many studies have re-evaluated the role of the inanimate environment in the epidemiology of infections caused by antibiotic resistant bacteria, for example Methicillin Resistant Staphylococcus aureus (MRSA), Vancomycin Resistant Enterococcus spp. (VRE), Clostridium difficile and Acinetobacter spp. that can survive for long periods of time on the surfaces of hospital rooms,3 operating rooms,4 autopsy rooms5 and intensive care units.6,7 Although the transmission of pathogens from one patient to another often occurs through the hands of the health care staff,8 water, air and surfaces can be involved, directly or indirectly. Like bacteria, some airborne virus (influenza viruses, respiratory syncytial virus, Adenovirus, Rhinovirus, Coronavirus, measles virus, rubella virus, mumps virus and human parvovirus B19) are transmitted by droplets that can be directly inhaled or deposited on the surfaces. Viral agents are also transmitted by oral-fecal route like the Rotavirus, human Adenovirus and Norovirus, that are frequently responsible of infections due to their presence in the air and on environmental surfaces.9 Adenovirus responsible for keratoconjunctivitis and gastroenteritis have been isolated from contaminated surfaces and instruments in various healthcare settings;10 the presence of viral nucleic acids such as Adenovirus and Norovirus, have been found in 16% of the total samples collected in hospital air and surfaces.11 Epidemics of Norovirus have also occurred in non-hospital environments, such as schools,12 military training centers,13 cruise ships14 and hotels.15 In these environments, the virus has been isolated from many different surfaces such as door handles, stair railings, rest rooms, toys, telephones, cups, materials, etc.9 Other studies have focused their attention on the role of the environmental surfaces in the transmission of bacteria from animals to humans and vice versa.16 The infections caused by human Papillomavirus (HPV type 7) are very common among workers in the meat slaughtering sector17 and among poultry farm workers.18 High concentrations of mesophilic bacteria (Bacillus cereus) and their components (endotoxins), fungi (Cladosporium spp., Mucor spp., Rhizopus oryzae, etc.) such as Aspergillus fumigatus, toxins, metabolites (microbial organic volatile compounds, MVOC) present in bioaerosol and on work surfaces in composting systems have been recognized as being responsible for several pathologies (chronic respiratory diseases, allergies, mucous membrane irritation, etc.) in the workers in this field.19 The risk of exposure to various pathogenic agents (Enterococcus spp, Escherichia coli, Klebsiella pneumoniae, Leptospira spp, Pseudomonas spp, Salmonella typhi, Shigella spp, Enterovirus, Rotavirus, Hepatitis viruses, Entamoeba histolitica, Giardia lamblia, Ascaris lumbricoides, etc.) present on work surfaces has been documented also among the workers of the waste water processing plants20,21 and among workers of the solid waste treatment sector.19,22 Finally, other studies23,24,25 have estimated the bacterial and fungal loads on different indoor environment surfaces (houses, stores, nurseries, offices, gymnasiums, restaurants, etc.). Elsergany et al. (2015) have found that 80% of the total of the 224 samples collected from the surfaces of 4 different shopping malls in Sharjah (United Arab Emirates) showed bacterial concentrations with high medium values (range between 500 and 1500 CFU/cm2).26 In libraries and archives,27 the fungal species most frequently isolated from books, manuscripts, documents, etc. have been Cladosporium herbarum, Cladosporium cladosporioides, Penicillium corylophilum, Aspergillus fumigatus, Penicillium spp., Aspergillus sydowii, Rhizopus nigricans.
Index/benchmark for occupational sectors
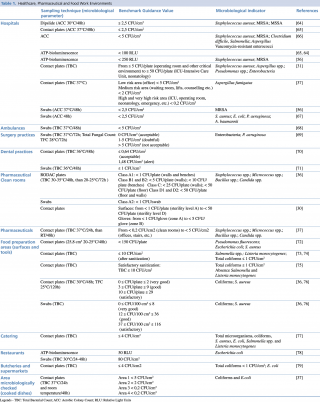
The literature on the quantitative evaluation of the levels of microbial contamination of the surfaces does not report standards or legislative references.62,63 The reported values are mostly finalized to estimate the cleaning efficacy of sanitation actions or to quantify and provide a general measure of bacterial load. Table 1 summarizes the benchmark values proposed by several authors in Healthcare, Pharmaceutical and Food work environments.
Table 1. Healthcare, Pharmaceutical and Food Work Environments
In the hospitals, the bacterial load considered as microbiological standard for the surfaces, is generally indicated between < 2,5 CFU/cm2 and < 5 CFU/cm2. The values of ATP bioluminescence indicating a clean surface ranged from < 250 RLU to 100 RLU. The index organisms that must be absent or <1 CFU/cm2 are mostly Staphylococcus aureus (including MRSA and MSSA), Aspergillus spp., Pseudomonas spp. and Enterobacteriaceae.
The Guide du Bionettoyage (Biocleaning Guide) n. 5670 ARECLIN and the ISPESL 2009 Guide Lines, divide the hospital workplaces into two\six zones characterized by different values of acceptability according with increasing risk. Different limits of acceptability occur for the pharmaceutical sector.37,31 For dentistry studies, a limit of acceptability of ≤ 1 CFU/cm2 had been proposed in 2008, while in 2012 an Italian multicenter study proposed threshold values based on the mean levels of the analyzed microbial accumulation.71,70
Microbiological contamination of foods can be ascribed to contaminated raw materials or cross-contamination events, caused by microorganisms originating from various sources, air, water, human or animal faeces, mucus, hair, infected wounds, dirt, dust. Mainly surface sampling is assessed with contact plates or swabs and further viable cell counting. A more rapid method, as the ATP bioluminescence, is used for the real-time evaluation of the cleanliness of food contact surfaces.
The effectiveness of cleaning and disinfection practices is often monitored by reductions of bacteria such as Salmonella, Salmonella, E. coli, Listeria monocytogenes and for total bacterial load, total coliform load. Proposed reference values for bacterial contamination in the food sector show a high variability compared to the sanitary sector. Risk management, reflected in the HACCP principle used by the food industry, encompasses the view that relevant pathogens are widespread, occurring with wide variation in time and space. This reasoning could be applied to surface level cleanliness in hospitals. Widespread adoption of standards would allow risk assessment and evaluation of infection risks to patients (and staff) in hospitals. Many papers propose acceptable values of mold contamination, lower than the bacterial ones for workplaces and houses.
Discussion
In the scientific literature and technical regulations there are many papers about the surface contamination in “Pharmaceutical-Sanitary” and “Food and Animal Feed” workplaces. In hospitals, surface contamination is an important source of potential pathogen microorganism. Correct cleaning systems and efficient disinfection of the surfaces reduces the incidence of the infections related to healthcare assistance, because the surfaces contamination has a principal role in the transmission of pathogenic microorganisms. Microbiological studies about air, water, (hydro-sanitary systems and air conditioning systems) and surfaces in various hospital environments are generally carried out for risk assessment and to establish monitoring actions. The total bacterial load and/or the pathogenic species responsible of nosocomial infections represent the microbial contamination indicators.
In ISPESL Guidelines31 and Annex I of EU GMP Guide30 are reported the reference values for the estimation of microbiological monitoring results respectively in the operating units and in the medicine industry.
Microbiological samplings are carried out mainly using the contact plates method, swabs, sponge-bag method and bioluminescence technique. Each single method shows both the advantages and limits of its use and the choice of the method to adopt is only conditioned by the surfaces to be examined.
Conclusion
This review shows the absence of standard operating procedure applicable to every workplace and the lack of threshold values for surface microbiological monitoring. We can only refer to index/benchmark proposed in literature, that are summarized in this paper. However, many different methods are indicated by the authors. This makes the comparison between the analytical results difficult because the protocols are characterized by different analytical parameters. Moreover, in the scientific literature there are not threshold limit for the microorganism indicators of the indoor air quality62,63 and the only found references are linked to the effectiveness of the sanitation actions. One adoptable proposal of procedures is present in the Inail Manual on microbiological Monitoring of the working environments.80
References
- Direttiva 2000/54/CE del Parlamento Europeo e del Consiglio del 18 settembre 2000 relativa alla protezione dei lavoratori contro i rischi derivanti da un'esposizione ad agenti biologici durante il lavoro (settima direttiva particolare ai sensi dell'articolo 16, paragrafo 1, della direttiva 89/391/CEE). Gazzetta ufficiale delle Comunità europee L 262/21 (2000).
- D. Lgs. 9 aprile 2008, n. 81. Attuazione dell’articolo 1 della Legge 3 agosto 2007, n. 123 in materia di tutela della salute e della sicurezza nei luoghi di lavoro. Gazzetta Ufficiale n. 101 del 30 aprile 2008 - Suppl. Ordinario n. 108.
- Chemaly, R.F., Simmons, S., Dale, C. Jr., Ghantoji, S.S., Rodriguez, M., Gubb, J., Stachowiak, J., Stibich, M.: The Role of the Healthcare Environment in the Spread of Multidrug-Resistant Organisms: Update on Current Best Practices for Containment. Ther Adv Infect Dis. 2(3-4), 79-90 (2014) doi: 10.1177/2049936114543287
- Yezli, S., Barbut, F., Otter, J.A.: Surface Contamination in Operating Rooms: A Risk for Transmission of Pathogens? Surg Infect (Larchmt) 15(6), 694-699 (2014). doi: 10.1089/sur.2014.011.
- Maujean, G., Malicier, D., Fanton, L.: Air, Water, and Surface Bacterial Contamination in a University-Hospital Autopsy Room. J Forensic Sci. Vol. 57, No.2 (2012).
- Russotto, V., Cortegiani, A., Raineri, S.M., Giarratano, A.: Bacterial Contamination of Inanimate Surfaces and Equipment in the Intensive Care Unit. J Intensive Care. 10;3, 54 (2015). doi: 10.1186/s40560-015-0120-5.
- Hu, H., Johani, K., Gosbell, I.B., Jacombs, A.S., Almatroudi, A., Whiteley, G.S., Deva, A.K., Jensen, S., Vickery, K.: Intensive Care Unit Environmental Surfaces Are Contaminated by Multidrug-Resistant Bacteria in Biofilms: Combined Results of Conventional Culture, Pyrosequencing, Scanning Electron Microscopy, and Confocal Laser Microscopy. J Hosp Infect. 91(1), 35-44 (2015). doi: 10.1016/j.jhin.2015.05.016.
- WHO. Guidelines on Hand Hygiene in Health Care. (2009).
- Rodríguez-Lázaro, D., Cook, N., Ruggeri, F.M., Sellwood, J., Nasser, A., Nascimento, M.S., D'Agostino, M., Santos, R., Saiz, J.C., Rzeżutka, A, Bosch, A., Gironés, R., Carducci, A., Muscillo, M., Kovač, K., Diez-Valcarce, M., Vantarakis, A., von Bonsdorff, C.H., de Roda Husman, A.M., Hernández, M., van der Poel, W.H.: Virus Hazards from Food, Water and Other Contaminated Environments. FEMS Microbiol Rev. 36(4), 786-814 (2012). doi: 10.1111/j.1574-6976.2011.00306.
- Dos Santos da Silva, J. V., de Mello, M. H., Staggemeier, R., Henzel, A., Rigotto, C., Spilki, F. R.: Adenovirus Presence in Surfaces and Equipment from Ambulatories, Internship Units, and Operating Rooms in a Brazilian Hospital. Letters to the Editor/American Journal of Infection Control. 42, 688-96 (2014).
- Carducci, A., Verani, M., Lombardi, R., Casini, B., Privitera, G.: Environmental Survey to Assess Viral Contamination of Air and Surfaces in Hospital Settings. Journal of Hospital Infection. 77, 242-247 (2011).
- CDC (Centers for Disease Control and Prevention). Norovirus Outbreaks on Three College Campuses – California, Michigan, and Wisconsin. MMWR Morb Mortal Wkly Rep 58, 1095–1100 (2009).
- Goodgame, R.: Norovirus Gastroenteritis. Curr Infect Dis Rep 9,102–109 (2007). doi 10.1007/s11908-007-0004-5.
- Isakbaeva, E.T., Widdowson, M.A., Beard R.S., Bulens, S.N., Mullins, J., Monroe, S., Bresee, J., Sassano, P., Cramer, E.H., Glass, R.I.: Norovirus Transmission on Cruise Ship. Emerg Infect Dis. 11(1), 154–157 (2005). doi: 10.3201/eid1101.040434.
- Kimura, H., Nagano, K., Kimura, N., Shimuzo, M.: A Norovirus Outbreak Associated with Environmental Contamination at a Hotel. Epidemiology & Infection Volume 139 Issue 2, 317-325 (2011). doi: 10.1017/S0950268810000981.
- Hanselman, B.A., Kruth, S.A., Rousseau, J., Weese, J.S.: Coagulase Positive Staphylococcal Colonization of Humans and Their Household Pets. Can Vet J 50, 954–958 (2009).
- Melchers, W., de Mare, S., Kuitert, E., Galama, J., Walboomers, J., van den Brule, A.J.: Human Papillomavirus and Cutaneous Warts in Meat Handlers. J Clin Microbiol. 31(9), 2547-2549 (1993).
- Mergler, D., Vezina, N. and Beauvais, A.: Warts Among Workers in Poultry Slaughterhouses. Scandinavian Journal of Work, Environment & Health Vol. 8 Supplement 1, 180-184 (1982).
- Nadal, M., Inza, I., Schuhmacher, M., Figueras, M.J., Domingo, J.L.: Health Risks of the Occupational Exposure to Microbiological and Chemical Pollutants in a Municipal Waste Organic Fraction Treatment Plant. Int. J. Hyg. Environ. Health 212(6), 661-669 (2009). doi: 10.1016/j.ijheh.2009.06.002.
- Viegas, C., Dias, R., Gomes, A.Q., Meneses, M., Sabino, R., Viegas, S.: Aspergillus Flavus Contamination in Two Portuguese Wastewater Treatment Plants. J Toxicol Environ Health A. 77(14-16), 796-805 (2014). doi: 10.1080/15287394.2014.909300.
- Sigari, G., Panatto, D., Lai, P., Stefani, L., Giuntini, A., Carducci, A., Gasparini, R.: Virological Investigation on Aerosol from Waste Depuration Plants. J. of Prev. Med. and Hyg. 47, 4-7 (2006).
- Viegas, C., Gomes, A.Q., Abegão, J., Sabino, R., Graça, T., Viegas, S.: Assessment of Fungal Contamination in Waste Sorting and Incineration-Case Study in Portugal. J Toxicol Environ Health A. 77(1-3), 57-68 (2014). doi: 10.1080/15287394.2014.86558.
- Bech-Andersen, J., Elborne, S.A.: Moulds and Indoor Climate in Denmark. In: 34th Annual Meeting, Brisbane, Australia (2003).
- Otter, J.A., French, G.L.: Bacterial Contamination on Touch Surfaces in the Public Transport System and in Public Areas of a Hospital in London. Lett Appl Microbiol. 49(6), 803-805 (2009). doi: 10.1111/j.1472-765X.2009.02728.x.
- Thapaliya, D., Taha, M., Dalman, M.R., Kadariya, J., Smith, T.C.: Environmental Contamination with Staphylococcus Aureus at a Large, Midwestern University Campus. Sci Total Environ. 599-600, 1363-1368 (2017). doi: 10.1016/j.scitotenv.2017.05.080. Epub 2017 May 15.
- Elsergany, M., Moussa, M., Ahsan, A., Khalfan, A., Eissa, A.: Exploratory Study of Bacterial Contamination of Different Surfaces in Four Shopping Malls in Sharjah, UAE. Journal of Environmental and Occupational Science. 4 (2), 101-105 (2015).
- Zielińska-Jankiewicz, K., Kozajda, A., Piotrowska, M., Szadkowska-Stanczyk, I.: Microbiological Contamination with Moulds in Work Environment in Libraries and Archive Storage Facilities. Ann. Agric. Environ. Med. 15, 71-78 (2008).
- ISO 14644-1 e 2:2015 – Cleanrooms and Associated Controlled Environments.
- ISO 14698-1 e 2:2003 – Cleanrooms and Associated Controlled Environments – Biocontamination Control.
- European Commission. Guidelines to Good Manufacturing Practice Medicinal Products for Human and Veterinary Use Revision to Annex 1. Manufacture of Sterile Medicinal Products. Brussels (2008).
- ISPESL – Dipartimento Igiene del Lavoro. Linee guida sugli standard di sicurezza e di igiene del lavoro nel reparto operatorio (2009).
- CCLIN Sud-Ouest: Guide de bonnes pratiques. Surveillance microbiologique de l’environnement dans les établissements de santé (2016).
- U.S. Department of Health and Human Services: Guidelines for Environmental Infection Control in Health-Care Facilities. Recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC). CDC (Centers for Disease Control and Prevention), Atlanta (2003).
- ISO 4833-1:2013 – Microbiology of the Food Chain. Horizontal Method for the Enumeration of Microorganisms.
- Istituto Zooprofilattico Sperimentale delle Venezie. Linee guida per il campionamento di superfici per analisi microbiologica. rev. 2, 1-7 (2015).
- ISO 18593:2004 – Microbiology of Food and Animal Feeding Stuffs. Horizontal Methods for Sampling Techniques from Surfaces Using Contact Plates and Swabs.
- ARECLIN Commission Centrale des Marchés. Guide du bionettoyage: recommandation n. E1-90. (1990).
- ISO 18593:2004 – Microbiology of Food and Animal Feeding Stuffs. Horizontal Methods for Sampling Techniques from Surfaces Using Contact Plates and Swabs.
- Ashour, M.S.E.D., Mansy, M.S., Eissa, M.E.: Microbiological Environmental Monitoring in Pharmaceutical Facility. Egypt. Acad. J. Biolog. Sci., 3(1), 63-74 (2011).
- Kearns, K.A., Witmer, D., Makda, J., Parvizi, J., Jungkind, D.: Sterility of the Personal Protection System in Total Joint Arthroplasty. Clin. Orthop. Relat. Res. 469, 3065-3069 (2011).
- Kac, G., Podglajen, I., Vaupre, S., Colardelle, N., Buu-Hof, A., Gutmann, L.: Molecular Epidemiology of Extended-Spectrum Beta-Lactamase Producing Enterobacteriaceae Isolated from Environmental and Clinical Specimens in a Cardiac Surgery Intensive Care Unit. Infect. Control Hosp. Epidemiol. 25, 852-855 (2004).
- Martirosian, G.: Recovery of Clostridium Difficile from Hospital Environments. J. Clin. Microbiol. 44(3), 1202-1203 (2006). doi: 10.1128/JCM.44.3.1202-1203.
- Eckstein, B.C., Adams, D., Eckstein, E., Rao, A., Sethi, A.K., Yadavalli, G.K., Donskey, C.J.: Reduction of Clostridium Difficile and Vancomycin-Resistant Enterococcus Contamination of Environmental Surfaces after an Intervention to Improve Cleaning Methods. BMC Infect. Dis. 7, 61 (2007).
- Carling, P.C., Briggs, J., Hylander, D., Perkins, J.: An Evaluation of Patient Area Cleaning in 3 Hospitals Using a Novel Targeting Methodology. Am. J. Infect. Dis. 42, 385-388 (2006).
- Hedin, G., Rynback, J., Lore B.: New Technique to Take Samples from Environmental Surfaces Using Flocked Nylon Swabs. Hosp Infect. 75(4), 314-317 (2010). doi: 10.1016/j.jhin.2010.02.027
- Rose, L., Jensen, B., Peterson, A., Banerjee, S.N., Arduino, M.J.: Swab Materials and Bacillus Anthracis Spore Recovery from Nonporous Surfaces. Emerg. Infect. Dis. 10(6), 1023-1029 (2004). doi:10.3201/eid1006.030716
- Hodges, L.R., Rose, L.J., Peterson, A., Noble-Wang, J., Arduino, M.J.: Evaluation of a Macrofoam Swab Protocol for the Recovery of Bacillus Anthracis Spores from a Steel Surface. Appl. Environ. Microbiol. 72, 4429-4430 (2006). doi: 10.1128/AEM.02923-05
- Lewandowski, R., Kozlowska, K., Szpakowska, M., Stepinska, M., Trafny, E.A.: Use of Foam Spatula for Sampling Surfaces after Bioaerosol Deposition. Appl. Environ. Microbiol. 76 (3), 688-694 (2010). doi: 10.1128/AEM.01849-09
- Landers, T.F., Hoet, A., Wittum, T.E.: Swab Type, Moistening, and Preenrichment for Staphylococcus Aureus on Environmental Surfaces. J. Clin. Microbiol. 48, 2235-2236 (2010).
- Saegeman, V., Flamaing, J., Muller, J., Peetermans, W.E., Stuyck, J., Verhaegen, J.: Clinical Evaluation of the Copan Eswab for Methicillin-Resistant Staphylococcus Aureus Detection and Culture of Wounds. Eur. J. Clin. Microbiol. Infect. Dis. 30, 943-949 (2011). doi:10.1007/s10096-011-1178-1
- Dolan, A., Bartlett, M., McEntee, B., Creamer, E., Humphreys, H.: Evaluation of Different Methods to Recover Meticillin-Resistant Staphylococcus Aureus from Hospital Environmental Surfaces. J. Hosp. Infect., 79, 227-230 (2011).
- Lejeune, B.: La contamination des surfaces de l’environnement hospitalier. Congres SFHH, Montpellier (2004).
- Garcia-Cruz, C.P., Najera Aguilar, M.J., Arroyo-Helguera, O.E.: Fungal and Bacterial Contamination on Indoor Surfaces of a Hospital in Mexico. Jundishapur J. Microbiol. 5(3), 460-464 (2012).
- Konecka-Martyjek, E., Mackiw, E., Krygier, B., Tomczuk, K., Stos, K., Jarosz, M.: National Monitoring Study on Microbial Contamination of Food-Contact Surfaces in Hospital Kitchen in Poland. Ann Agric Environ Med. 19 (3), 457-463 (2012).
- Rose, L.J., Hodges, L., O’ Connell, H., Noble-Wang, J.: National Validation Study of a Cellulose Sponge Wipe-Processing Method for Use after Sampling Bacillus anthracis Spores from Surfaces. Appl. Environ. Microbiol. 77(23), 8355-8359 (2011). doi: 10.1128/AEM.05377-11
- Sherlock, O., O’Connell, N., Creamer, E., Humphreys, H.: Is It Really Clean? An Evaluation of the Efficacy of Four Methods for Determining Hospital Cleanliness. J. Hosp. Infect. 72, 140-146 (2009).
- Kyriakides, A.L., Costello, S.M., Doyle, G., Easter, M.C., Johson, I.: Rapid Hygiene Monitoring Using ATP Bioluminescence in: Stanley P.E., Kricka, L.J.: Bioluminescence and Chemiluminescence: Current Status. 519-522. John Wiley and Sons, Chichester, (1991).
- Davidson, C.A., Griffith, C.J., Peters, A.C., Fielding, L.M.: Evaluation of Two Methods for Monitoring Surface Cleanliness – ATP Bioluminescence and Traditional Hygiene Swabbing. Luminescence 14(1), 33-38 (1999).
- Dumigan, D.G., Boyce, J.M., Havill, N.L., Golebiewsky, M., Balogun, O., Rizvani, R.: Who Is Really Caring for Your Environment of Care? Developing Standardized Cleaning Procedures and Effective Monitoring Techniques. American Journal of Infection Control 38, 387-392 (2010). doi:10.1016/j.ajic.2009.07.005
- Ceresa, L.: ATP Bioluminescenza: benefici di un metodo di microbiologia. In: Atti Congresso Tecnologie di determinazione rapida. Firenze (2009).
- Istituto Superiore di Sanità. Linee guida per l’analisi microbiologica dei prodotti cosmetici, Rapporti Istisan 13/15 (2013).
- Dacarro, C., Grignani, E., Lodola, L., Grisoli, P., Cottica, D.: Proposta di indici microbiologici per la valutazione della qualità dell’aria degli edifici. G. It. Med. Lav. Erg. 22(3), 229-235 (2000).
- European Commission. European Collaborative Action. Indoor Air Quality and Its Impact on Man. Report N. 12. Biological Particles in Indoor Environments. (1993).
- Mulvey, D., Redding, P., Robertson, C., Woodall, C., Kingsmore, P., Bedwell, D., Dancer, S.J.: Finding a Benchmark for Monitoring Hospital Cleanliness. J. Hosp. Infect. 77, 25-30 (2011).
- Amodio, E., Cannova, L., Villafrate, M.R., Merendino, A.M., Aprea, L., Calamusa, G.: Analytical Performance Issues: Comparison of ATP Bioluminescence and Aerobic Bacterial Count for Evaluating Surface Cleanliness in an Italian Hospital. J. Occ. Environ. Hyg. 11(2), D23-D27 (2014).
- Dancer, S.J.: The Role of Environmental Cleaning in the Control of Hospital-Acquired Infection. J Hosp Infect. 73(4), 378-385 (2009).
- Huang, P.Y., Shi, Z.Y., Chen, C.H., Den, E., Huang, H.M., Tsai, J.J.: Airborne and Surface-Bound Microbial Contamination in Two Intensive Care Units of a Medical Center in Central Taiwan. Aerosol Air Qual. Res. 13, 1060–1069 (2013).
- Luksamijarulkul, P., Pipitsangjan, S.: Microbial Air Quality and Bacterial Surface Contamination in Ambulances During Patient Services. Oman Med. J. 30(2), 104–110 (2015). doi: 10.5001/omj.2015.23
- Catamo, G., Di Bonaventura, G., Lattanzio, F.M., Lattanzio, D., Piccolomini, R.: Monitoraggio microbiologico di aria e superfici in ambiente di prime cure chirurgiche di ambulatorio INAIL. G. It. Micro. Med. Odont. Clin. vol III(2), 128 – 134 (1999).
- Pasquarella, C., Veronesi, L., Napoli, C., Castiglia, P., Liguori, G., Rizzetto, R., Torre, I., Righi, E., Farruggia, P., Tesauro, M., Torregrossa, M.V., Montagna, M.T., Colucci, M.E., Gallè, F., Masia, M.D., Strohmenger, L., Bergomi, M., Tinteri, C., Panico, M., Pennino, F., Cannova, L., Tanzi, M.: Microbial Environmental Contamination in Italian Dental Clinics: A Multicenter Study Yielding Recommendations for Standardized Sampling Methods and Threshold Values. Sci Total Environ. 420, 289–299 (2012). doi: 10.1016/j.scitotenv.2012.01.030.
- Castiglia, P., Liguori, G., Montagna, M.T., Napoli, C., Pasquarella, C., Bergomi, M., Fabiani, L., Monarca, S., Petti, S., SItI Working Group Hygiene in Dentistry: Italian Multicenter Study on Infection Hazards During Dental Practice: Control of Environmental Microbial Contamination in Public Dental Surgeries. BMC Public Health 8, 187 (2008). doi: 10.1186/1471-2458-8-187.
- Silverman, G.J., Ross, E.W., Kautz, W.P.: Assessment of the Sanitary Quality of Food Preparation Surface. Journal of Foodservice System 4, 285-301 (1981).
- L. 30 aprile 1962 n. 283 - Disciplina igienica della produzione e della vendita delle sostanze alimentari e delle bevande, G.U. n. 139 del 4 giugno 1962.
- Commission Regulation (EC) n. 2073/2005 of 15 November 2005 on Microbiological Criteria for Foodstuffs.
- Osimani, A., Garofalo, C., Clementi, F., Tavoletti, S., Aquilanti, L.: Bioluminescence ATP Monitoring for the Routine Assessment of Food Contact Surface Cleanliness in a University Canteen. Int. J. Environ. Res. Public Health 11, 10824-10837 (2014). doi: 10.3390/ijerph111010824.
- ISO/TC 34/SC 9 doc N 374: Project for ISO 18593 “Enumeration of Aerobic Bacteria Using Contact Plates or Dipslide and Swabs Methods”. (1999).
- Garayoa, R., Diez-Leturia, M., Bes-Rastrollo, M., Garcia-Jalon, I., Vitas, A.I.: Catering Services and HACCP: Temperature Assessment and Surface. Food Control 43, 193-198 (2014).
- Azizkhan, Z.M.: Comparison Between ATP Bioluminescence Technique and Traditional Microbiological Method to Detect Contamination within Food Facilities in Saudi Arabia (Jiddah). Front. Public Health 3(1), 11-18 (2014).
- Souliotis, A., Palisidis, G., Giazitzi, K., Boskou, G.: Benchmarking the Hygiene of Utensils in Butcheries or Retail Stores. SAJ Nutri Food 1, 101 (2015).
- INAIL: Il monitoraggio microbiologico negli ambienti di lavoro. Campionamento e analisi (2010).