In vitro experimental models to study the efficiency of the placental barrier for environmental toxicants: tumor cell lines versus trophoblast primary cells.
Luisa Campagnolo, Valentina Lacconi, Micol Massimiani, Andrea Magrini and Antonio Pietroiusti
Department of Biomedicine and Prevention, School of Medicine and Surgery, University of Rome “Tor Vergata”, Rome, Italy
Introduction
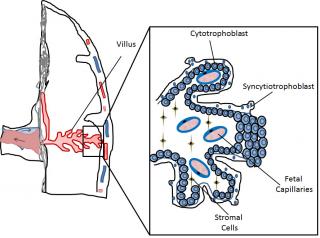
Very early during mammalian development the embryo differentiates into 2 main cell lineages, the inner cell mass (ICM) which will mainly contribute to embryonic tissues, and the trophoblast which will form extra-embryonic tissues. The trophoblast represents an evolutionary innovation, contributing to the formation of the “connection” between the mother and the fetus, namely the placenta, with no further need of great accumulation of nutrients and mRNA in the egg yolk. Several differences exist in placental organization among different mammalian species, making it difficult to automatically transpose toxicological results from common in vivo models, such as rodents, to humans. In humans the placental barriers are composed by the endothelium of the fetal capillaries, and two layers of trophoblast cells, the syncytiotrophoblast, composed of syncytia forming a continuous lining of the villous tree, and the cytotrophoblast, composed of single cells forming a discontinuous layer in the mature placenta (Figure 1).
Figure 1. Schematic representation of part of the human placenta and magnification of a placental villus depicting its cellular organization.
Considering the ethical limitations for fetal toxicity studies resulting from maternal exposure to different environmental toxicants in humans, the development of complex in vitro models, which considers the placental barrier as well as the developing fetal tissues, appears of great importance. Ex vivo models have been proposed as an alternative for translocation studies, however they exploit the use of term placentas, which after delivery remain viable for a very limited time. In addition, lack of reproducibility and standardizations represent main limitations of this model.1
In this mini-review, we aim to present and critically analyse the different in vitro models that have been proposed as an alternative to in vivo and ex vivo studies to elucidate the ability of different xenobiotics to interfere with fetal development by direct (transplacental crossing and accumulation in embryonic tissues) or indirect action (e.g., inflammation, perturbation of placental function).
The Trophoblast Cell Lines
Several trophoblast cell lines have been developed for in vitro studies of trophoblast physiology and in the following paragraphs we will give a brief overview on the cells have been used to develop in vitro models of the placental barrier.
Choriocarcinoma cells
Several trophoblast cell lines have been developed and used to reproduce in vitro the trophoblast layer of the placenta. Many of these cell lines have been obtained from a highly metastatic malignant tumor of the trophoblast, the choriocarcinoma, and have been extensively characterized. The BeWo cell line is one of the most widely used for translocation studies, as its ability to form a barrier with low permeability has been reported.2-4 In particular, a clone of the BeWo cells, BeWo b30, shows a good ability to form syncytia upon stimulation with forskolin, an activator of adenylyl cyclase, better resembling the syncytiotrophoblast layer of the placenta. BeWo cells have been used to study transport and metabolism of drugs, including opioid peptides,2,5 the tranplacental crossing of engineered nanoparticles6 and of bacteria.7 Other choriocarcinoma cell lines widely used to study placental permeability to xenobiotics are the Jeg-3 and Jar. Very recently, these three cell lines have been exploited in a comparative study on functional parameters. The reported data indicated a great variability in glucose transport, human chorionic gonadotropin (hCG) release and nanoparticle internalization and transfer, which could not simply be ascribed to differences in cell proliferation or metabolism.4 As an attempt to better resemble features of trophoblast cells, the ACH-3P trophoblast cell line has been established by fusion of primary human first trimester trophoblasts (gestation week 12) with the AC1-1 human choriocarcinoma cell line.8 Comparative analysis with the above mentioned choriocarcinoma cell lines shows that ACH-3P cells display a transfer ratio similar to what reported in ex vivo placenta perfusion studies, suggesting that this cells might represent a good alternative for transport studies in vitro; however, ACH-3P cells poorly respond to forskolin treatment and do not form syncytia, lacking the possibility to give rise to the syncytiotrophoblast layer of the placenta, which is one of the main component of the placental barrier. All the above mentioned limitations point toward the need of alternative methods based on the use of non-cancer cells.
Non-cancer trophoblast cells
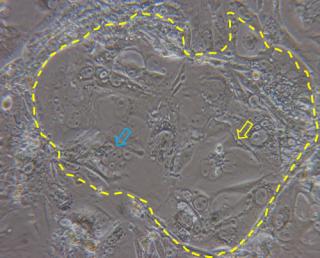
A possible alternative to cancer cells is the use of primary trophoblast cells purified from first trimester placentas obtained after elective terminations of pregnancy or from term placentas. One big limitation is, however, that these cells are hardly obtained in great numbers and proliferate very little in culture. In an attempt to overcome such limitation, primary trophoblast cells have been purified from first trimester human villi (HTR-8) and have been transfected with the gene encoding simian virus 40 large T antigen in order to extend their lifespans (HTR-8/SVneo). The comparison between transformed and parental cells showed no major morphological differences, no signs of tumor formation after subcutaneous injection in nude mice, and expression of cytokeratin marker, confirming the epithelial identity of the cells. Differently from the parental cells, which senesced after 12 passages, the HTR-8/SVneo cells could be maintained in culture over 32 passages, allowing their prolonged use for in vitro studies.9 However, HTR-8/SVneo have been never used for translocation studies, possibly due to their extravillous trophoblast origin and reduced propensity to form syncytia. In this respect, trophoblast stem cells (TSCs) represent a valid alternative. TSCs can be fairly easily obtained from mouse blastocysts10 and more recently their derivation from human villous trophoblast cells has been reported.11 Mouse TSCs have been used for in vitro molecular and functional studies on trophoblast lineage differentiation, and it has been demonstrated that they can give rise to all different type of trophoblast cells of the mouse placenta, including the syncytiotrophoblast.12-14 Syncytiotrophoblast differentiation does not need the addition of drugs and can be simply obtained by removal of heparin and FGF4 from the culture medium (Figure 2). Due to their reproducibility, easy derivation and versatile differentiation ability, these cells appear as promising in vitro tools to study placental barrier function.
Figure 2. Dashed line highlights a colony of TSCs cultured for three days in basal medium (without heparin and FGF4) in which syncytialized structures are evident (yellow arrow) together with cluster of cells in the process of forming syncytia (blue arrow).
In Vitro Models of the Placental Barrier
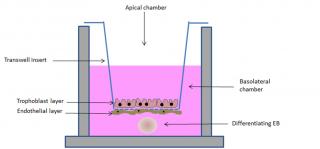
Several models have been proposed for studying in vitro the translocation potential of xenobiotics across the placental barrier, from complex multi-layered microfluidic systems,15 to spheroids16 and transwell models.17 Although, on one hand, complexity is desirable to more faithfully reproduce the organ, on the other hand standardization and easy manipulation seem of great importance. In this respect, the transwell model appears promising. In this model system, trophoblast cells, mainly the BeWo clone30b, are cultured on polycarbonate porous membrane cell culture inserts, where they are able to form polarized monolayers.3 Recently, nine selected model compounds have been tested for their ability to be transported from the apical to the basolateral compartment of this simulated placental barrier and the comparison with data obtained from ex vivo studies indicate a good correlation of the transfer indices.18 In an attempt to include the other cell types involved in the formation of the placental barrier, Aengenheister and colleagues introduced in the model endothelial cells.19 Human placental venous endothelial cells can be cultured on the side of the transwell insert opposite to the one where trophoblast cells are later plated (Figure 3), making the simulated barrier better resembling the in vivo condition. This model has been tested for the translocation of placental crossing drugs, such as antipyrine, and of hydrophilic substances (Na-F and FITC-dextran) and polystyrene nanoparticles of different sizes. Results indicate a good ability of the simulated barrier to shield translocation of the tested compounds, excepting the antipyrine.
In order to simulate in vitro not just the placental barrier, but the more complex feto-placental unit, we are currently developing a model which includes, beside the placental cell lines, a simulated embryo. To this end, we have exploited the ability of embryonic stem (ES) cells to form embryoid bodies (EBs) in 3D cultures. EBs recapitulate in vitro embryonic development, differentiating into derivatives of the three germ layers.20,21 Such a differentiation ability has been exploited in the embryonic stem cell test (EST), a test validated by the ECVAM (European Committee for the Validation of Alternative Methods) for the evaluation of the embryotoxic potential of a wide group of chemical compounds.22 We have demonstrated that the EST can be used to investigate the embryotoxic potential of engineered nanoparticles.23 However, the lack of a simulated placental barrier limits the application of the test as cannot discriminate the ability of xenobiotic to reach the fetus and to cause either direct or indirect toxicity. In our new model, the “barrier EST” (bEST) schematically represented in Figure 3, we have included the EBs (to represent the embryo) in the transwell model (representing the placental barrier). As an additional improvement, we have used TSCs grown in differentiating conditions to form the syncytiotrophoblast layer on the transwell insert membrane. Our preliminary results show an efficient ability of the simulated barrier to reduce the embryotoxic effect of some NPs, evaluated as reduced interference with the differentiation program of the EBs.
Figure 3. Schematic representation of the bEST in vitro model. A porous transwell culture insert, on which syncytiotrophoblast and endothelial cells (to simulate the placental barrier) are seeded, separates two compartments: an apical compartment, in which the tested compound is added, and a basolateral chamber, in which a differentiating embryoid body (EB, to resemble the embryo) is cultured.
Conclusion and Open Questions
According to the European Legislation, animal studies should be highly limited. Beside ethical questions, it is also of importance to carefully assess how an animal model can represent human physiology. For specific tissues and organs, e.g. the placenta, structural interspecies differences exist, limiting the translation of results obtained in one species to another. In vitro models might represent a valid alternative. With respect to transport studies across the placental barrier, the ethical limits can be overcome by the use of such in vitro models. Over the last two decades much work has been done in this direction. The recent development of human TSCs and the availability of human ES cell lines allow to set up our proposed in vitro model using exclusively human derived cells. Although different techniques can be used to assess the formation and integrity of the barrier, and the physiological expression of molecules involved in the regulation of transcellular transport, as for example transmission electron microscopy analysis, transepithelial resistance measurements and gene expression analysis, no direct comparison with the in vivo studies is possible, leaving opened the question on how much the in vitro data can be transposed to humans. One possible solution could come from an in depth analysis of epidemiological data. A focus on this would be desirable.
References
- Berveiller P, Gil S, Vialard F. Placental Perfusion: Interest and Limits. J Matern Fetal Neonatal Med. 2017 Jun;30(11):1347-1348.
- Wice B, Menton D, Geuze H, Schwartz AL (1990) Modulators of Cyclic AMP Metabolism Induce Syncytiotrophoblast Formation In Vitro. Exp Cell Res 186:306–316.
- Liu F, Soares MJ, Audus KL. Permeability Properties of Monolayers of the Human Trophoblast Cell Line BeWo. Am J Physiol. 1997 Nov;273(5 Pt 1):C1596-604.
- Rothbauer M, Patel N, Gondola H, Siwetz M, Huppertz B, Ertl P. A Comparative Study of Five Physiological Key Parameters between Four Different Human Trophoblast-Derived Cell Lines. Sci Rep. 2017 Jul 19;7(1):5892.
- Ampasavate C, Chandorkar GA, Vande Velde DG, Stobaugh JF, Audus KL. Transport and Metabolism of Opioid Peptides Across Bewo Cells, An In Vitro Model of the Placental Barrier. Int J Pharm. 2002 Feb 21;233(1-2):85-98.
- Correia Carreira S, Walker L, Paul K, Saunders M. The Toxicity, Transport and Uptake of Nanoparticles in the In Vitro Bewo B30 Placental Cell Barrier Model Used Within NanoTEST. Nanotoxicology. 2015 May;9 Suppl 1:66-78.
- Lecuit M, Nelson DM, Smith SD, Khun H, Huerre M, Vacher-Lavenu MC, Gordon JI, Cossart P. Targeting and Crossing of the Human Maternofetal Barrier by Listeria Monocytogenes: Role of Internalin Interaction with Trophoblast E-Cadherin. ProcNatl Acad Sci U S A. 2004 Apr 20;101(16):6152-7
- Preli Hiden U, Wadsack C, Prutsch N, Gauster M, Weiss U, Frank HG, Schmitz U, Fast-Hirsch C, Hengstschläger M, Pötgens A, Rüben A, Knöfler M, Haslinger P, Huppertz B, Bilban M, Kaufmann P, Desoye G. The First Trimester Human Trophoblast Cell Line ACH-3P: A Novel Tool to Study Autocrine/Paracrine Regulatory Loops of Human Trophoblast Subpopulations--TNF-Alpha Stimulates MMP15 Expression. BMC Dev Biol. 2007 Dec 19; 7:137.
- Graham CH, Hawley TS, Hawley RG, MacDougall JR, Kerbel RS, Khoo N, Lala PK. Establishment and Characterization of First Trimester Human Trophoblast Cells with Extended Lifespan. Exp Cell Res. 1993 Jun;206(2):204-11.
- Tanaka S, Kunath T, Hadjantonakis AK, Nagy A, Rossant J. Promotion of Trophoblast Stem Cell Proliferation by FGF4. Science. 1998 Dec 11;282(5396):2072-5
- Okae H, Toh H, Sato T, Hiura H, Takahashi S, Shirane K, Kabayama Y, Suyama M, Sasaki H, Arima T. Derivation of Human Trophoblast Stem Cells. Cell Stem Cell. 2018 Jan 4;22(1):50-63. e6.
- Arul Nambi Rajan K, Khater M, Soncin F, Pizzo D, Moretto-Zita M, Pham J, Stus O, Iyer P, Tache V, Laurent LC, Parast MM. Sirtuin1 Is Required for Proper Trophoblast Differentiation and Placental Development in Mice. Placenta. 2018 Feb; 62:1-8.
- Zhu D, Gong X, Miao L, Fang J, Zhang J. Efficient Induction of Syncytiotrophoblast Layer II Cells from Trophoblast Stem Cells by Canonical Wnt Signaling Activation. Stem Cell Reports. 2017 Dec 12;9(6):2034-2049.
- You JL, Wang W, Tang MY, Ye YH, Liu AX, Zhu YM. A Potential Role of Galectin-1 in Promoting Mouse Trophoblast Stem Cell Differentiation. Mol Cell Endocrinol. 2017 Nov 6. pii: S0303-7207(17)30568-3. doi: 10.1016/j.mce.2017.11.003.
- Blundell C, Tess ER, Schanzer AS, Coutifaris C, Su EJ, Parry S, Huh D. A Microphysiological Model of the Human Placental Barrier. Lab Chip. 2016 Aug 2;16(16):3065-73.
- Muoth C, Aengenheister L, Kucki M, Wick P, Buerki-Thurnherr T. Nanoparticle Transport Across the Placental Barrier: Pushing the Field Forward! Nanomedicine (Lond). 2016 Apr;11(8):941-57.
- Cartwright L, Poulsen MS, Nielsen HM, Pojana G, Knudsen LE, Saunders M, Rytting E. In Vitro Placental Model Optimization for Nanoparticle Transport Studies. Int J Nanomedicine. 2012; 7:497-510.
- Li H, van Ravenzwaay B, Rietjens IM, Louisse J. Assessment of an In Vitro Transport Model Using Bewo B30 Cells to Predict Placental Transfer of Compounds. Arch Toxicol. 2013 Sep;87(9):1661-9.
- Aengenheister L, Keevend K, Muoth C, Schönenberger R, Diener L, Wick P, Buerki-Thurnherr T. An Advanced Human In Vitro Co-Culture Model for Translocation Studies Across the Placental Barrier. Sci Rep. 2018 Mar 29;8(1):5388.
- Itskovitz-Eldor J, Schuldiner M, Karsenti D, Eden A, Yanuka O, Amit M, Soreq H, Benvenisty N. Differentiation of Human Embryonic Stem Cells into Embryoid Bodies Compromising the Three Embryonic Germ Layers. Mol Med. 2000 Feb;6(2):88-95
- Choi D, Lee HJ, Jee S, Jin S, Koo SK, Paik SS, Jung SC, Hwang SY, Lee KS, Oh B. In Vitro Differentiation of Mouse Embryonic Stem Cells: Enrichment of Endodermal Cells in the Embryoid Body. Stem Cells. 2005 Jun-Jul;23(6):817-27.
- Genschow E, Spielmann H, Scholz G, Seiler A, Brown N, Piersma A, Brady M, Clemann N, Huuskonen H, Paillard F, Bremer S, Becker K. The ECVAM International Validation Study on In Vitro Embryotoxicity Tests: Results of the Definitive Phase and Evaluation of Prediction Models. European Centre for the Validation of Alternative Methods. Altern Lab Anim. 2002 Mar-Apr;30(2):151-76.